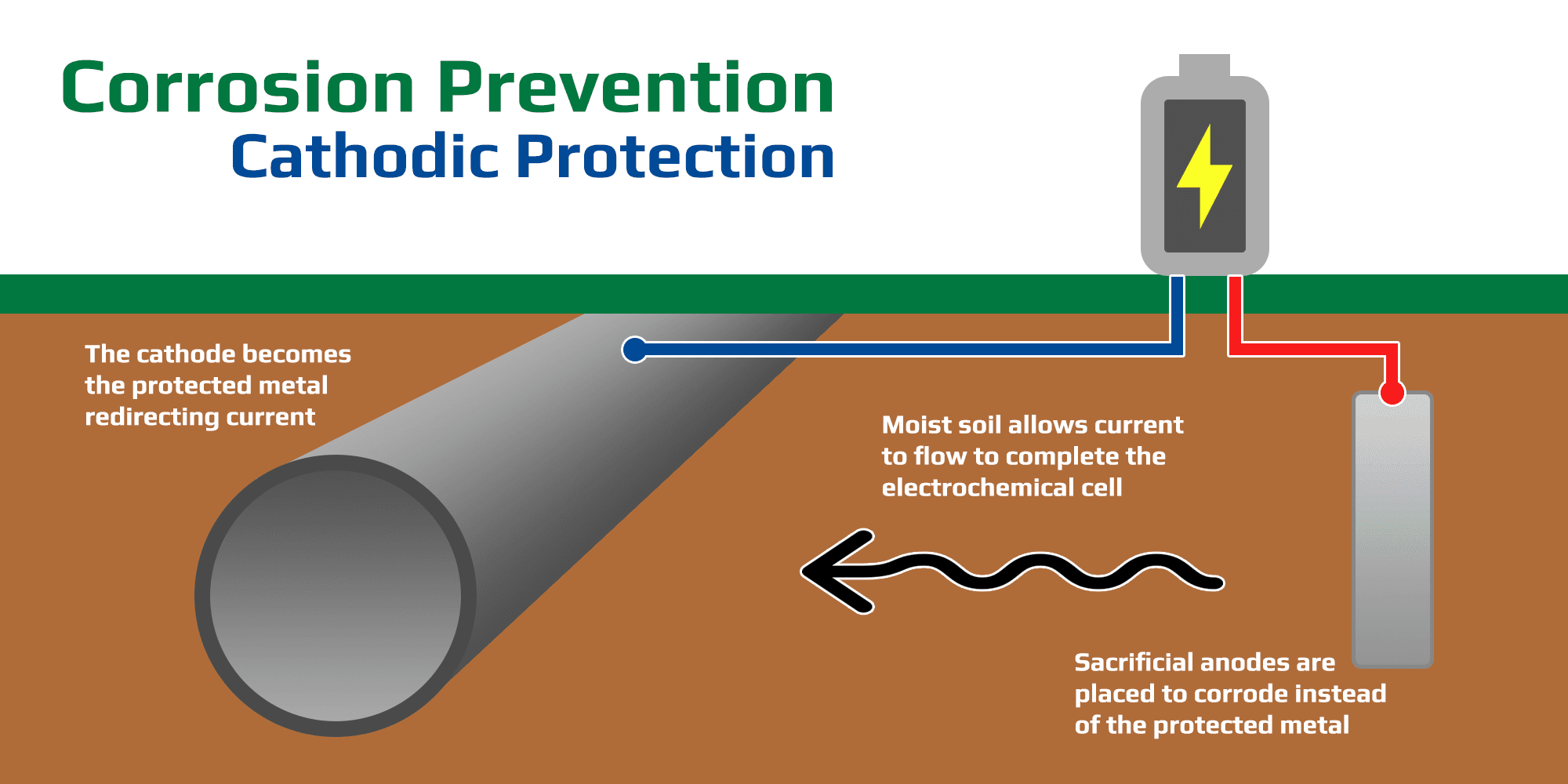
Cathodic protection prevents metal corrosion effectively.
Anodic protection suits specific acidic environments.
Cost-efficient for pipelines, ships, tanks.
Why Cathodic Protection Matters
Cathodic protection (CP) shields metals like steel in harsh environments, preventing costly damage and safety risks in industries like oil and gas.
How Cathodic Protection Works
CP makes metal a cathode, supplying electrons to halt oxidation:
Sacrificial Anodes: Zinc or magnesium corrodes instead.
Impressed Current: External power delivers continuous current.
Anodic Protection
Anodic protection applies positive potential, forming a passive oxide layer on metals like stainless steel in acids, reducing corrosion.
Benefits
Extends asset life.
Lowers maintenance costs.
Enhances safety.