Info
Corrosion Process
2024.05.03
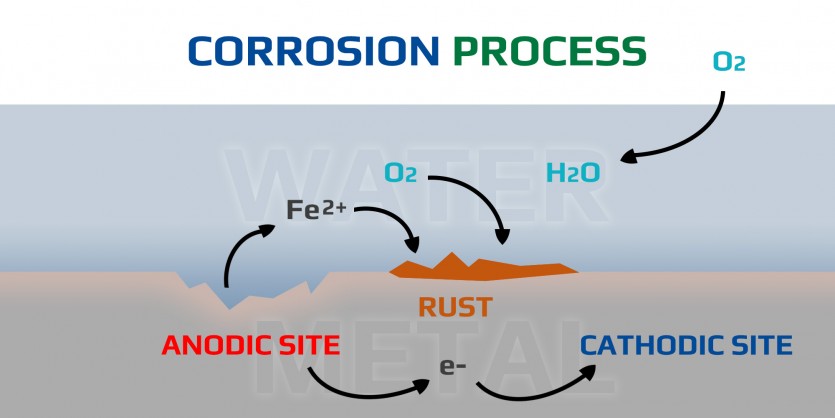
In the corrosion process, ferrous ions (Fe2+) are produced when iron metal undergoes oxidation reactions(anodic sites), losing electrons to its surroundings(cathodic sites). These ferrous ions then react with water(H2O) and oxygen(O2) in the environment to form hydrated iron oxides, commonly known as rust. The presence of ferrous ions in the solution facilitates further corrosion reactions by participating in electrochemical processes, leading to the continued breakdown of the metal surface. As the corrosion progresses, more ferrous ions are released into the solution, perpetuating the cycle of oxidation and reduction. The accumulation of rust and the continuous generation of ferrous ions contribute to the ongoing degradation of the metal structure over time.

 EN
EN